Key takeaways:
- Developing software for medical devices requires a structured approach that balances technical precision, regulatory compliance, and real-world usability to ensure patient safety and device reliability.
- There is a wide variety of medical devices ranging from wearable sensors to smart hospital beds to mobile and desktop apps classified as a device.
- Compared to off-the-shelf solutions, custom medical devices provide you with more control and flexibility in terms of regulatory compliance, data security mechanisms, and device performance.
- Developing software for medical devices requires a combination of skills in software development, cybersecurity, integration, and cloud connectivity.
Medical devices have become an integral part of healthcare-related businesses, with a market valued at $519 billion in 2023 and projected to grow from $887 billion by 2032. Despite numerous benefits and use cases, these devices pose a pool of challenges with device management, performance, integrations with IT systems, and data security.
Developing custom software for medical devices helps companies solve these challenges and adapt the devices to their unique needs. In this article, we examine key benefits and use cases for custom devices, and provide medical device software development tips based on our expertise in embedded development, cybersecurity, and cloud computing.
This article is intended for in healthcare companies and organizations looking to make their devices more efficient and secure.
Contents:
What is a medical device?
Determining whether a product is a medical device may seem simple, but in fact, the term “medical device” isn’t limited to devices in the general sense of the word like smartwatches and pacemakers. It covers a variety of things that can be used for medical tasks and use cases.
Here’s how the World Health Organization defines medical devices:
There are an estimated 2 million different kinds of medical devices on the world market, categorized into more than 7,000 generic device groups.
A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use, software, material, or other similar or related article, intended by the manufacturer to be used alone or in combination for a medical purpose.
World Health Organization
The US Food and Drug Administration provides an even broader definition, calling a medical device anything that includes a component that can be called a medical device.
Since the use of medical devices and software for them is heavily regulated by healthcare and cybersecurity laws, requirements, and standards, it’s important to classify your product correctly before you start development. Here’s how to determine if your product is a medical device:
- Check product classification databases
- Analyze the use cases for your product
- Take a look at common types of medical devices
- Research whether your competitors market their products as medical devices
The classification of medical device software depends on its role, integration level, and regulatory definitions. While different sources may categorize them differently, the most common types include the following:
- Embedded medical system software (EMSSW) is software running on specialized hardware within a medical device like a pacemaker or ventilator control system. This type of software and firmware must ensure real-time operation, precise device control, and secure communication.
- Software in a medical device (SiMD) is somewhat similar to EMSSW. SiMD is software that is an integral part of a medical device but may run on general-purpose hardware. For instance, imaging software in MRI machines or infusion pump control applications can work both on-device and remotely.
- Software as a medical device (SaMD) is standalone software that performs medical functions without being tied to a specific piece of hardware. Examples include AI-powered diagnostic tools and mobile applications for disease monitoring.
- Medical device software (MDSW) is a broad definition encompassing any software that directly impacts a device’s medical function. As a legal term, MDSW is only used in the EU.
In this article, we’ll use the more general definition of medical device, meaning “some type of physical device”.
For medical devices, software is a critical component that affects accuracy, efficiency, and data protection capabilities. In the next section, we’ll take a look at the key use cases for such software.
Plan on powering your devices with custom software?
Leverage Apriorit’s expertise in custom embedded software development, integrations, and cybersecurity to build the exact solution you need.
Use cases for medical device software
Whether embedded in hardware, operating as a standalone application, or functioning within a larger healthcare IT ecosystem, medical device application development enhances business processes, thus improving patient outcomes. These are the key use cases for software that powers medical devices:
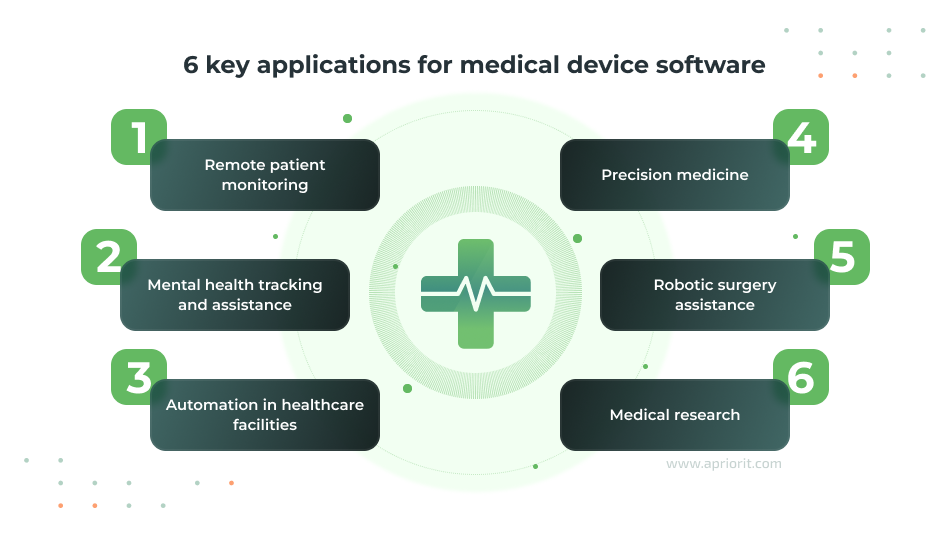
1. Remote patient monitoring
Medical devices can collect real-time data on a patient’s vital signs and pass it to a centralized hospital database, helping doctors make informed decisions while tracking patient health outside of healthcare facilities. These devices can include smart glucose meters, wearable pulse and oxygen saturation meters, remote EGCG monitors, ingestible sensors, etc. For each of these, the software is responsible for collecting, processing, and securely transmitting patient data.
For example, device firmware can encrypt and transmit records of a patient’s heart rate to a hospital in real time, or a mobile app connected to a smart inhaler can help a patient track their usage and define triggers for asthma attacks.
When applied to this use case, medical device software can help reduce hospital admission rates, lighten the load for physical healthcare facilities, provide better care to patients who can’t access a traditional hospital, and give patients with chronic illnesses tools to manage their conditions.
2. Mental health tracking and assistance
Device software for mental health usually includes SaMD products like mobile apps that guide cognitive behavioral therapy sessions. SaMD helps care professionals monitor physiological and behavioral indicators that reflect a patient’s well-being. Patients can also use them to track and better understand and manage their conditions.
This use case can also include physical medical devices like wearable EEG headbands that measure brainwave activity to help track mood fluctuations or smart rings and wristbands that monitor and track sleep cycles and stress responses. Software processes this data to identify patterns that might signal anxiety, depression, or other mental health conditions.
3. Automation in healthcare facilities
Healthcare professionals and administrators are overrun with administrative work, barely finding the time to provide quality care to their patients. Device-driven automation helps them outsource trivial tasks to devices and focus on complex cases that require human interaction.
One example of this use case is automated medication dispensers that control precise dosing, freeing up the time of medical personnel and reducing the risk of human error. In such dispensers, device firmware, integrated into a hospital’s EHR system, manages medicine stock and controls dispensing mechanisms. Another example is the use of smart hospital beds equipped with software that adjusts patient positioning to prevent bedsores, monitors vital signs, and alerts staff if a patient is at any risk.
Read also
Using Automation Anywhere to Create Bots for Task Automation: A Practical Example
We overview how Automation Anywhere works for different tasks. Read on to learn how to implement it and automate repetitive administration activities inside your business.
4. Precision medicine
Taking traditional medicine one step further, precision medicine helps doctors quickly analyze patient information that was previously unavailable to tailor treatment plans to individual patients. This approach relies heavily on software and hardware to analyze complex biological data and guide clinical decisions.
When applied in precision medicine, medical device software usually:
- Collects data from multiple sources (genomic, clinical, imaging)
- Transmits it to a centralized data management system
- Participates in pattern recognition
Devices supporting precision medicine include genomic sequencing platforms that analyze DNA to identify genetic mutations linked to diseases. These devices can, for example, analyze a tumor’s genetic profile to help the doctor recommend personalized treatment plans with the highest likelihood of success.
5. Robotic surgery assistance
Hospitals rely heavily on sophisticated robots to enhance precision, control, and visualization during surgery procedures. Surgical robots are equipped with arms controlled by surgeons via software interfaces that provide real-time feedback, precise control, and high-definition 3D views of the surgical site.
The device’s software coordinates its movements, translating the surgeon’s hand motions to guide surgical instruments. Some systems feature AI-driven guidance tools that suggest optimal incision sites or warn against potential risks during the procedure. Additionally, the software logs surgical data for post-operative analysis. These records help improve patient outcomes and are useful for medical training.
6. Medical research
Collecting and managing information in a fast and secure way is one of the key challenges of medical research. Researchers need data from wearables, imaging and screening systems, organs-on-a-chip, and other devices to be streamed into their research management solutions and databases without delays and errors. At the same time, it’s important that medical device software handles such tasks while maintaining data safety and anonymity.
These devices are invaluable for clinical trials and patient monitoring as they help researchers to:
- Avoid human error when inputting and processing data
- Automate data collection
- Speed up research and trial processes
Related project
Developing Drivers for Low Latency Virtual Reality Headsets
Learn the story of a professional VR headset manufacturer who needed help with custom drivers for their device. With our custom code, their headsets now work in low-latency mode for robotic surgeries and pilot training.
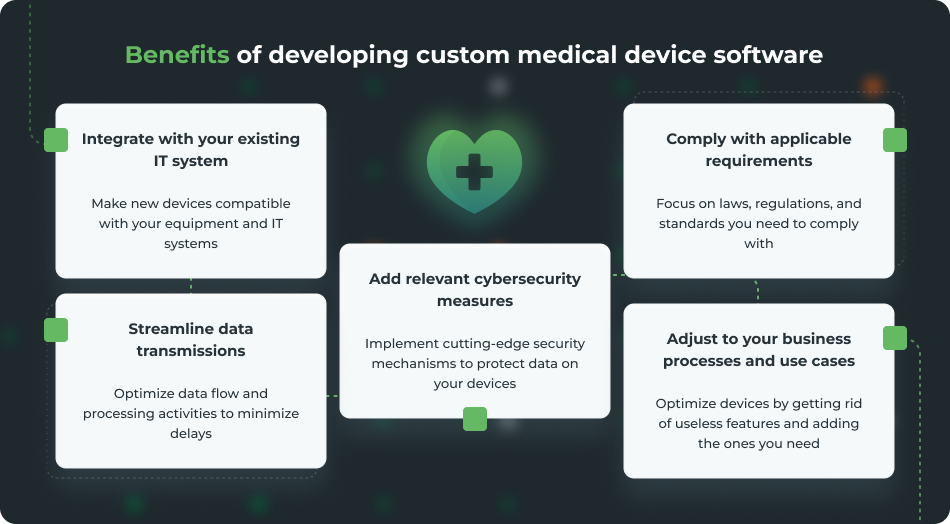
How can custom software improve medical devices?
Off-the-shelf medical device software may offer convenience, but it often falls short of meeting healthcare organizations’ specific needs. A custom-built solution for operating, managing, and securing medical devices allows your organization to:
How to develop medical device software
Software engineering for medical devices follows a general SDLC flow with some tweaks. You can adjust these development stages according to your business processes and software requirements. For example, you can outsource some coding and QA activities to a dedicated vendor or conduct additional checks to ensure the needed levels of software security and performance are met.
Here are the key development steps to follow:
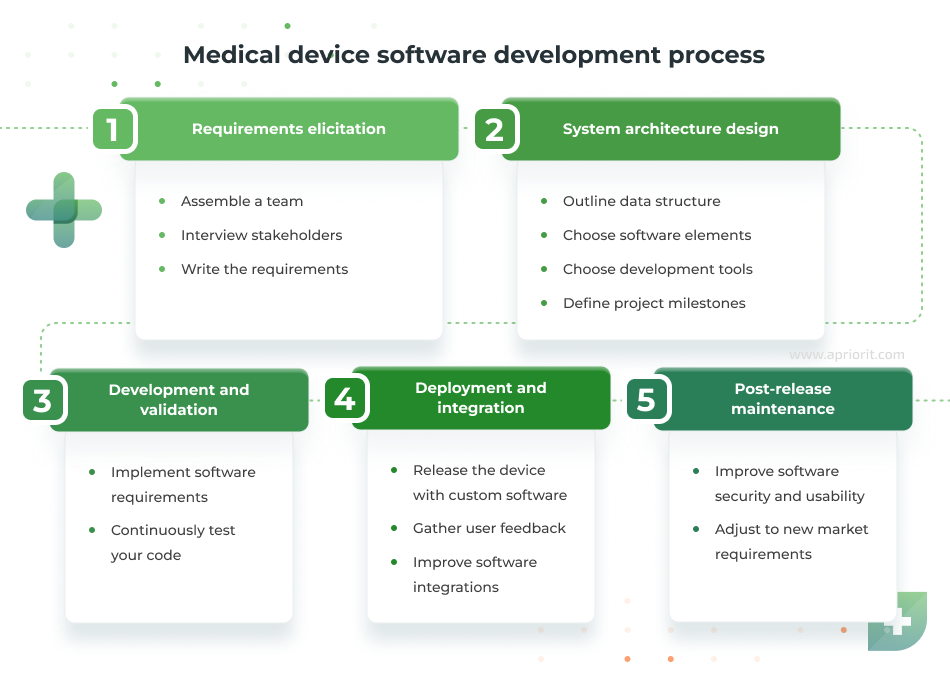
- Requirements elicitation. This initial stage of the project is dedicated to defining the software’s purpose, functionality, and safety standards. Usually, this stage involves business analysts, software developers, and QA specialists. They gather input from stakeholders like healthcare professionals and device manufacturers and compile project requirements. Clear requirements help to define the vision of your product, plan the development process, and keep the project team on the same page.
- System architecture design. Based on the gathered requirements, engineers outline the software’s structure, data flow, interactions with hardware components, communication protocols, and so on. Thoughtful architecture planning allows you to build a scalable, secure, and reliable system that supports both current and future needs.
- Development and validation. Developers focus on implementing functional and non-functional requirements gathered at previous stages. Validation usually happens in parallel, as the QA team looks for performance, security, compatibility, and usability issues. Your team can use virtual testing tools to check software security and performance, but it’s best if you can provide them with physical devices for real-life testing.
- Deployment and integration. Once validated, the software is integrated with the medical device hardware and deployed in clinical or operational environments. This stage includes final system testing to verify compatibility in the real world, introduce necessary fixes, and gather user feedback.
- Post-release maintenance. Device support and continuous improvement are indispensable steps for ensuring the security and commercial success of your product in the long run. After deployment, monitor for performance issues, security vulnerabilities, and opportunities for improvement. Software updates may address new regulatory requirements, introduce enhancements, or fix bugs.
Whether you decide to develop in-house or hire a dedicated vendor, you can customize this general workflow with personal tweaks to satisfy your vision of the product and specific requirements. In the next section, we’ll discuss nuances our medical device software developers consider when building healthcare solutions.
Read also
Adopting Generative AI in Healthcare: Key Benefits, Use Cases, and Challenges
GenAI is taking many industries, including healthcare, by storm. Learn from our AI development experts what you can expect from this technology, when you may need it, and how to prepare for its adoption.
What to consider when developing software for medical devices
Developing software for medical devices is a complex process that goes beyond just coding a working solution. It requires balancing technical precision, regulatory compliance, and real-world usability. It is also very nuanced and depends heavily on many factors, including:
- The type of medical device you want to power
- Applicable laws, regulations, and standards
- The environment you want to integrate your device in
- Preferences of your customers and end users
Based on our experience with healthcare products and embedded development, we’ve outlined these key considerations for medical device software.
1. Ensure your compliance with relevant requirements
As part of the healthcare system, medical device software is governed by many laws, standards, and regulations. In addition to general documents like HIPAA and the GDPR, research relevant regulations for your region and the type of device you want to power. Here are the key documents you’ll want to check:
| Market | Enforcement | Focus | |
|---|---|---|---|
| HIPAA | US and partners of US-based healthcare companies | Mandatory | Privacy and security of any form of healthcare data |
| GDPR | EU and companies that work within the EU/with EU citizens | Mandatory | Privacy and security of personal information |
| IEC 62304 | International | Not mandatory, often requested | Best practices for medical software development life cycle |
| ISO 14971 | International | Not mandatory, often requested | Risk management for medical devices (hardware and software) |
| FD&C Act | US | Mandatory | Risk classification and management of medical devices and software |
| MHRA regulations | UK | Mandatory | Device and software documentation, certification, and marketing |
2. Implement reliable data security
Medical device software collects, processes, and transmits sensitive data constantly. Securing this data must be one of the key focus points during development. One way to ensure reliable security is to follow the guidelines and compliance requirements, which usually include data security measures. On top of that, you can implement best practices for embedded device security and network protection like the following:
- Strong encryption for data in transit and at rest. Implement protocols like TLS 1.3 or AES-256 to protect data during transmission between devices, cloud servers, and hospital systems.
- Authentication and access control for any data managed by your software. It must be able to check the rights of any users, software products, and other devices.
- Continuous activity logging. This feature is necessary for device monitoring, analyzing errors and malfunctions, and reviewing possible security incidents.
- A vulnerability management system for deployed devices. Your software should include continuous security patches, remote data and device management, and periodic security testing sessions or independent audits.
3. Check integration efficiency
Medical devices are always operating within a system of healthcare IT solutions: other devices, personal applications, networks, EHR and hospital management systems, etc. Ensuring interoperability between these systems can be challenging because of different data standards, protocols, and architectures. While standards like HL7 and FHIR aim to simplify this, inconsistent implementations can create compatibility issues.
Real-time data synchronization adds another layer of complexity. Embedded devices often don’t have enough computing resources to store the data they collect and stream it in real time. Records of some devices, like patient monitors or infusion pumps, also have to be streamed instantly to allow doctors to immediately respond to any changes.
To remedy this, developers can implement middleware solutions that act as translators between systems, ensuring data is consistently formatted and accurately mapped across platforms. Leveraging APIs with standardized data models also helps maintain compatibility. Optimized communication protocols like MQTT or WebSockets help to reduce latency in data transmission, which is useful for real-time communication.
4. Leave traces during software development
Traceability is one of the most common mandatory requirements for the development process of medical device software. Medical devices are a complicated type of product with multiple modules, dependencies, and third-party components. Maintaining clear documentation that links requirements to implementation, testing, and validation, can be a challenge, especially when teams are distributed or working on rapid development cycles. However, without traceability of the development process, it’s challenging to audit software, pass certifications, manage vulnerabilities, and identify the root causes of security and performance issues.
To address this, you can generate a software bill of materials (SBOM). These documents track components, modules, and third-party libraries used during development, helping to identify and patch vulnerabilities caused by external dependencies. You can also automate some traceability tasks with application lifecycle management tools that track code changes and link them to product requirements, test cases, and other documents.
5. Add advanced features and technologies
Integrating AI, data analytics, cloud computing, and other technologies can help you pack more cutting-edge features inside your software and significantly enhance its competitiveness. However, medical devices are often based on IoT chips and don’t have the computing power to run advanced code.
One way to solve this challenge would be a distributed architecture where a deployed device collects data and sends it to a remote server or a cloud service for processing. This type of architecture is only suitable for devices that don’t need to work in real time.
Cloud connectivity introduces additional scalability and security challenges. Increased connectivity expands the attack surface for cybersecurity threats, necessitating advanced security protocols that don’t compromise performance. To tackle these issues, developers can adopt modular software architectures that support scalability and flexibility.
Read also
Developing and Supporting a CRM System for a Medical Transportation Company
Explore how a US-based client recruited our team to take their CRM system to a new level with custom features and improved security measures.
How Apriorit can help with medical device software development
Partnering with an experienced software development vendor allows you to outsource all coding challenges and focus on other elements of your product. As a company with experience in medical device software development, we can help you with that.
Here’s what you get when you collaborate with Apriorit:
Our recent healthcare projects include:
- Building a custom AI-based model for scientific report analysis which works with over 92.8% accuracy.
- Developing drivers for a low-latency VR headset used to train healthcare professionals.
- Delivering an AI solution for object recognition in ultrasound images that detects, measures, and tracks ovarian follicles with 90% precision and a 97% recall rate.
- Helping a medical transportation company support and improve its internal CRM system, enhancing corporate data security and service quality.
- Conducting security testing for an iOS application that healthcare professionals use for remote visits.
Whether you want to build new custom software for medical devices, improve an existing product, or request a third-party security or code audit, we can provide you with a suitable team or individual experts for your situation.
Conclusion
Successful software development for medical devices requires a strategic approach that considers both development and clinical nuances. When implemented skillfully, custom software can help healthcare professionals improve service quality, provide care for more patients, and make data-driven medical decisions that will help improve patient outcomes.
Apriorit’s skilled and dedicated team will master the right balance between secure and fast performance, regulatory compliance, and real-world usability of your device software, giving your team time to focus on your product’s value for healthcare companies.
Want to power your device with custom software?
Apriorit experts can help you boost the intelligence of your business by implementing cutting-edge AI technologies. We provide AI development services to companies in various industries, from healthcare and education to cybersecurity and remote sensing.



